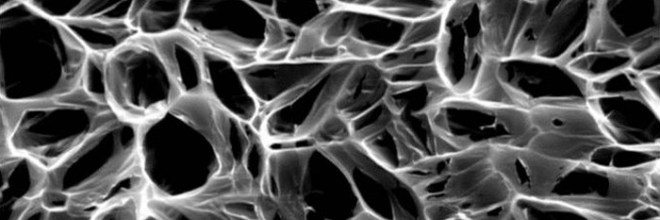
Carbon nanotubes serve as bridges that permit electrical signals to pass unhindered through new pediatric heart-defect patches invented at Rice University and Texas Children’s Hospital.
A team led by bioengineer Jeffrey Jacot and chemical engineer and chemist Matteo Pasquali come up with patches infused with conductive single-walled carbon nanotubes. The patches are constructed with a sponge-like bioscaffold that contains microscopic pores and mimics your body’s extracellular matrix.
The nanotubes overcome a limitation of current patches in which pore walls hinder the change in electrical signals between cardiomyocytes, the center muscle’s beating cells, which take up residence in the patch and finally change it with new muscle.
The work appears this month within the American Chemical Society journal ACS Nano. They said their invention could serve as a full-thickness patch to correct defects due to Tetralogy of Fallot, atrial and ventricular septal defects and other defects with no risk of inducing abnormal cardiac rhythms.
The original patches developed by Jacot’s lab consist primarily of hydrogel and chitosan, a widely used material produced from the shells of shrimp and other crustaceans. The patch is mounted on a polymer backbone that may hold a stitch and it in position to cover a hole within the heart. The pores allow natural cells to attack the patch, which degrades as the cells form networks that belongs to them. The patch, such as the backbone, degrades in weeks or months as it is substituted with natural tissue.
Researchers at Rice and elsewhere have found that when cells take their place in the patches, they’ve difficulty synchronizing with the remainder from the beating heart because the scaffold mutes electrical signals that pass from cell to cell. That temporary loss of signal transduction results in arrhythmias.
Nanotubes can deal with that, and Jacot, who has a joint appointment at Rice and Texas Children’s, took advantage of the surrounding collaborative research environment.
“This stemmed from speaking with Dr. Pasquali’s lab as well as interventional cardiologists in the Texas Medical Center,” Jacot said. “We’ve been surfing for a way to get better cell-to-cell communications and were focusing on the rate of electrical conduction with the patch. We thought nanotubes could be easily integrated.”
Nanotubes enhance the electrical coupling between cells that attack the patch, helping them keep up with the heart’s steady beat. “When cells first populate an area, their connections are immature compared with native tissue,” Jacot said. The insulating scaffold can delay the cell-to-cell signal further, but the nanotubes forge a path around the obstacles.
Jacot said the relatively low power of nanotubes – 67 parts per million in the patches that tested best – is essential. Earlier tries to use nanotubes in heart patches employed much higher quantities and different methods of dispersing them.
Jacot’s lab found a component these were already using within their patches C chitosan C keeps the nanotubes spread out. “Chitosan is amphiphilic, meaning it has hydrophobic and hydrophilic portions, so it can associate with nanotubes (that are hydrophobic) and keep them from clumping. That is what allows us to use much lower concentrations than others have tried.”
Because the toxicity of carbon nanotubes in biological applications remains a wide open question, Pasquali said, the less one uses, the better. “We want to stay at the percolation threshold, and obtain into it using the fewest nanotubes possible,” he explained. “We can do this if we control dispersion well and use high-quality nanotubes.”
The patches start as a liquid. When nanotubes are added, the mixture is shaken through sonication to disperse the tubes, which may otherwise clump, due to van der Waals attraction. Clumping might have been an issue for experiments that used higher nanotube concentrations, Pasquali said.
The materials are spun inside a centrifuge to eliminate stray clumps and formed into thin, fingernail-sized discs with a biodegradable polycaprolactone backbone that allows the patch to be sutured into place. Freeze-drying sets how big the discs’ pores, which are large enough for natural heart cells to infiltrate and for nutrients and waste to feed.
As a side benefit, nanotubes also result in the patches stronger minimizing their tendency to swell while providing a handle to exactly tune their rate of degradation, giving hearts lots of time to replace all of them with natural tissue, Jacot said.
“If there is a hole within the heart, a patch needs to take the full mechanical stress,” he explained. “It can’t degrade too fast, but it also can’t degrade not fast enough, since it would end up becoming scarring. You want to avoid that.”
Pasquali noted that Rice’s nanotechnology expertise and Texas Medical Center membership offers great synergy. “This is a good example of methods it’s far better for an application person like Dr. Jacot to utilize pros who understand how to handle nanotubes, instead of trying to go solo, as many do,” he explained. “We get a much better charge of the material. The converse often happens, obviously, and working with leaders within the biomedical field can definitely accelerate the road to adoption of these new materials.”
Seokwon Pok, a Rice research scientist in Jacot’s lab, is lead author of the paper. Co-authors are research scientist Flavia Vitale, graduate student Omar Benavides and former postdoctoral researcher Shannon Eichmann, all of Rice. Pasquali is chair of Rice’s Department of Chemistry along with a professor of chemical and biomolecular engineering, of materials science and nanoengineering as well as chemistry. Jacot is an assistant professor of bioengineering at Rice, director from the Pediatric Cardiac Bioengineering Laboratory in the Congenital Heart Surgery Service at Texas Children’s as well as an adjunct assistant professor at Baylor College of drugs.
The National Institutes of Health, the Welch Foundation and Texas Children’s Hospital supported the study.
Read the abstract at http://pubs.acs.org/doi/abs/10.1021/nn503693h

































